Red light triggers a change of lifestyle for ancient bacteria
By Natalia Bateman, CoETP, December 8, 2017
In a surprising discovery, scientists have found that an ancient marine bacteria changes from a lonely free-floating lifestyle to a sedentary communal life style, when it senses an environment rich on red light.
The bacteria, called Acaryochloris marina, has a special type of pigment – chlorophyll d – that permits these organisms to absorb light just beyond the red end of the visible spectrum, an area that plants can’t use.
Chlorophyll is the molecule responsible for capturing sunlight, so it can be transformed into food, through the process of photosynthesis.
The team of scientists have been researching what triggers the production of different types of chlorophyll in different organisms.
“The goal of our experiment was to investigate if the synthesis of different types of chlorophyll depended on the availability of different types of light. To our surprise, we found that this not the case in A. marina, and that red light doesn’t affect which pigment they use, but instead triggers the expression of genes that are related to the formation of bacterial communities called biofilms,” said Dr Miguel Hernandez, a researcher at the ARC Centre of Excellence for Translational Photosynthesis, at the University of Sydney.
Biofilms are communities of different types of bacteria. As examples, think about cambucha or the plaque that forms in your teeth. Far-red light triggers the expression of genes that produce an outer layer of sugar that helps the community members to attach to each other and form a biofilm.
“We are trying to understand how pigments, like chlorophyll, behave in different environments” says Professor Min Chen, Chief Investigator of the ARC Centre of Excellence for Translational Photosynthesis at the University of Sydney, and one of the authors of this study.
“From all the sunlight that falls on a plant just 4-7% is captured by chlorophylls a and b to use during photosynthesis. Imagine the possibilities if we could use a wider area of the light captured to produce food, by transferring other types of chlorophyll into crops. One day, our findings will permit other people to get these pigments into crop plants to increase their crop yield,” she said.
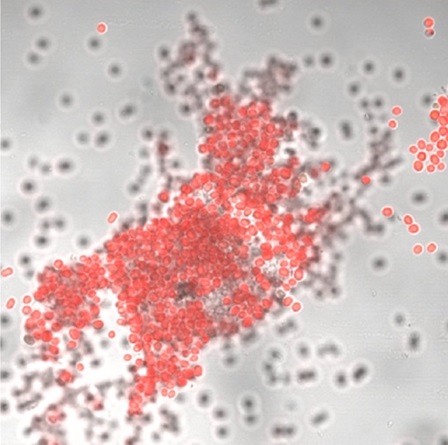
The scientists grew the bacteria under white and red light expecting to find changes in the expression of genes for the synthesis of different types of chlorophyll. However, they found that one of the most affected categories of gene expression was membrane generation.

The cells grown under far-red light (right) adhered to the walls and formed clumps while cells grown under a fluorescence white light were loose.
“Prior to our study, it was not known how A. marina identified these ecological niches that are rich in red light. Our results demonstrate that these environments induce the expression of genes that facilitate the adhesion of the bacteria to the surface,” said Dr Hernandez.
In nature, this bacteria grows forming biofilms with organisms that use chlorophyll a, so A. marina uses the leftovers of light from its neighbours. This gives A marina an advantage in places with low light and lots of available nutrients, where the bacteria is protected from the damaging burning effects of white light.
“Our study shows that the production of chlorophyll d is independent of the light conditions where you grow the bacteria, which means that the synthesis of chlorophyll d doesn’t require the presence of far-red light. These give us important information for future possibilities that involve putting these genes into crops, that generally grow under white light conditions,” he said.
This study was published recently in the journal Environmental Microbiology. This work is a collaboration between Washington State University and the Australian Research Council (ARC) Centre of Excellence for Translational Photosynthesis at the University of Sydney.

